Dragon Tree Consortium
Dragon Trees
Definition of Dragon tree group
Dragon trees are one of the most famous groups of trees in the world, well known from ancient times. Their distinctive habit, adaptation to arid condition, and especially the red resin (dragon’s blood) that they exude have had a long effect on human imagination and curiosity. Only a few species among more than 60–190 species of the genus Dracaena reach arborescent form. Members of the dragon tree group are usually defined by a few general traits, which are: arborescent habit, excretion of the red resin, leaving a closely packed pattern on the bark, closely packed leaves at branch apices with a lack of distinctive internodes, leaf sheaths differentiated in colour, very succulent leaves with a large amount of a water tissue and fibers, leaf blades lacking a distinctive costa, thick cuticle on the leaves, flowers usually diurnal with a perianth not elongated into a tube, and the filaments of the stamens inserted at the tepal bases with thickened filaments. The species considered as belonging to the dragon tree group are: Dracaena cinnabari Balf.f., D. draco L. subsp. draco, D. draco subsp. caboverdeana Marrero Rodr. & R. Almeida, D. draco subsp. ajgal Benabid & Cuzin, D. tamaranae A. Marrero, R. S. Almeida et M. González-Martín, D. ombet Heuglin ex Kotschy & Peyr., D. ombet subsp. schizantha Baker, D. serrulata Baker, and D. ellenbeckiana Engl. present in Macaronesia, North Africa, and South Arabia. The other species that are recognized as dragon trees are found in South East Asia: D. jayniana Wilkin & Suksathan, D. cochinchinensis (Lour.) S. C. Chen, D. yuccifolia Ridl., D. cambodiana Pierre ex Gagnep., and D. kaweesakii Wilkin & Suksathan.

Significance of Dragon tree group
The dragon tree group is significant for many different reasons, the most important of which are:
-
Dragon trees are tertiary relict species, and the ecosystems (woodlands and very rare forests) being of middle Oligocene to Miocene origin to which these species belong to are one of the evolutionary oldest ecosystems in the world.
-
Some species are locally endemic with limited (often island) distribution.
-
The distribution of most species is scattered, comprising a small population with unbalanced age structure, and young trees often bring absent.
-
Some species are threatened and are listed in the IUCN Red List. In fact, of the six species that have been assessed, five are threatened, while the other 11 species have not yet been assessed.
-
The natural regeneration of most species is being destroyed by decades of overgrazing.
-
Dragon trees are strongly susceptible and vulnerable to the effects of climate change (drought, cyclones).
-
Most species have been from ancient times as an important source of non-timber products (resin, fodder for cattle, and forage for bees, leaves used for rope production), thus belong to the cultural heritage of humanity.
-
Dragon trees are flagship and umbrella species hosting many other organisms, including endemic taxa, which are totally dependent on dragon trees.
-
Dragon trees strongly and positively influence the hydrology cycles in the landscape.
-
Most species are present in developing countries with an unstable political situation, and populations are under pressure, as weak governments have insufficient resources to protect them.
-
Dragon trees often provide local income through ecotourism by being aesthetically attractive species, popular with visitors. In addition, some species are commonly sold as attractive indoor plants.

History – dragon’s blood
Dragon’s blood is the name given to a deep red exudate obtained originally from Dracaena cinnabari and later from other arborescent dragon trees. From ancient times it was highly valued by many cultures for its traditional medicinal properties. Due to the belief that it is blood from a dragon, it was used also in magic rituals and alchemy. The chemical composition of resin in Dracaena species is very complex. Numerous phytochemical studies of resins from the Dracaena species resulted in the isolation and subsequent identification of sterols, steroidal saponins, lignans, terpenoids, flavonoids, bioflavonoids, carotenoids, and aromatic compounds. Dragon´s blood was used for its antiseptic properties and the resins are still a natural remedy in some cultures today. It was also a highly valued red dye. The first reference to the actual origins of dragon’s blood comes from the Periplus Maris Erythraei (mid-first century BC), which mentions that Arab, Indian, and Greek settlers were sailing to Socotra Island to trade in tortoise shell, aloes, and Indian cinnabar. According to Pliny the Elder’s Historia Naturalis, cinnabar is the name given to dragon’s blood by the Indians. Presumably the term Indian cinnabar, used to describe the dragon’s blood resin from Socotra, was due to it having been mostly distributed by Indian merchants or on Indian ships. Whilst the trade in dragon’s blood resin continued throughout the medieval period, there were an increasing number of alternatives from different geographical locations, such as the Canary Islands and the East Indies.
Individual species (description – morphology, ecology, distribution, utilisation)
Macaronesian group
The species of the Macaronesian group (D. dracos. l. and D. tamaranae) grow on the volcanic islands of Macaronesia and in the adjacent area of southwest Morocco. The area of Macaronesia encompasses several Atlantic archipelagos and a part of northwest African mainland, and spans over different climate types. Generally, in Macaronesia, temperature increases, and precipitation decreases towards the equator. The broad climate of each site is further modified by local conditions, mainly by orography, exposition, distance from the sea, and orientation to prevailing northeastern trade winds. Along with the differences, the habitats of Macaronesian dragon trees are largely similar in their environment, biogeography, and plant communities. The dragon trees in the area, likewise the species from the other two groups, prefer sites that are affected by fog formation. Here, the umbrella-shaped crowns with their dense leaf-rosettes intercept droplets of water from fog, which then drips and flows down the trees, enriching the soil with moisture.
Dracaena draco subsp. draco (L.) L.
Wild populations of D. draco subsp. draco are only known from Tenerife, it is considered to be extinct in wild in Gran Canaria and others Canary islands. Tenerife Island hosted 694 wild specimens. The current populations of D. draco subsp. draco in the rest of the archipelago are all of cultivated origin. This species also occurs in the Madeira archipelago, where it has been reported from the islands of Madeira and Porto Santo, although it is currently regarded as extinct in the wild too. There seems to be small remnant population on Azores, Flores Island. Cultivated, we can find this species in many places across the world. On Madeira, the wild population of D. draco subsp. draco was mainly found on sea-facing cliffs and as an element of thermo-sclerophyllous zones between sea-level and 200 m a.s.l. in the vegetation of the class Rhamno crenulatae–Oleetea cerasiformis. Similarly, on Tenerife, D. draco subsp. draco occurs in thermo-sclerophyllous vegetation of the same class, namely in the alliance Mayteno canariensis–Juniperion canariensis, between 100 and 600 m a.s.l., where the annual mean rainfall ranges between 200 and 400 mm. On Gran Canaria, D. draco subsp. draco grows in similar habitats to those on Tenerife, but only in the northeastern part of the island that is by the moisture-bearing trade winds.

Dracaena draco subsp. caboverdeana (Marrero Rodr. & R. S. Almeida) Rivas Mart., Lousã, J.C.Costa & Maria C. Duarte
D. draco subsp. caboverdeana was found on the Cape Verde Islands, on São Nicolau, Santo Antão, and Fogo. It is considered to be extinct on the islands of São Vicente and São Tiago [79], as growing sub-spontaneously on Santiago and Brava, and as cultivated on all islands. On the Cape Verde Islands, the climate with natural populations of D. draco subsp. caboverdeana is classified as tropical xeric and tropical pluviseasonal. The tree grows in a wide range of elevation from 50 m a.s.l. to 1400 m a.s.l. on the slopes that are exposed to the northeastern trade winds, which bring the majority of moisture to the archipelago. The characteristic community with D. draco subsp. caboverdeana is deciduous micro-woodland climactic savanna of the alliance Fico gnaphalocarpae–Acacion caboverdeanae, which was typically structured by short trees including the Dracaena. However, over the last centuries the demand for wood, grazing and agriculture has reduced the tree cover substantially.

Dracaena draco subsp. ajgal Benabid & Cuzin
D. draco subsp. ajgal, which is the most abundant (several thousand individuals). D. draco subspecies, is native to the western Anti-Atlas Mountains in Morocco, where it is restricted to rocky and steep cliffs of an isolated basin between two mountain ranges of the Jebel Imzi area. The elevation of the locality ranges from 250 m a.s.l. to 1540 m a.s.l. and the climate is characterized as infra-mediterranean in lower elevations to thermo-mediterranean in higher elevations. The mean annual temperature is estimated to be around 20O C and the annual rainfall to be at least 500 mm with additional moisture from frequent fogs. The communities with D. draco subsp. ajgal were classified into the association Davallio canariensis–Dracaenetum ajgal.
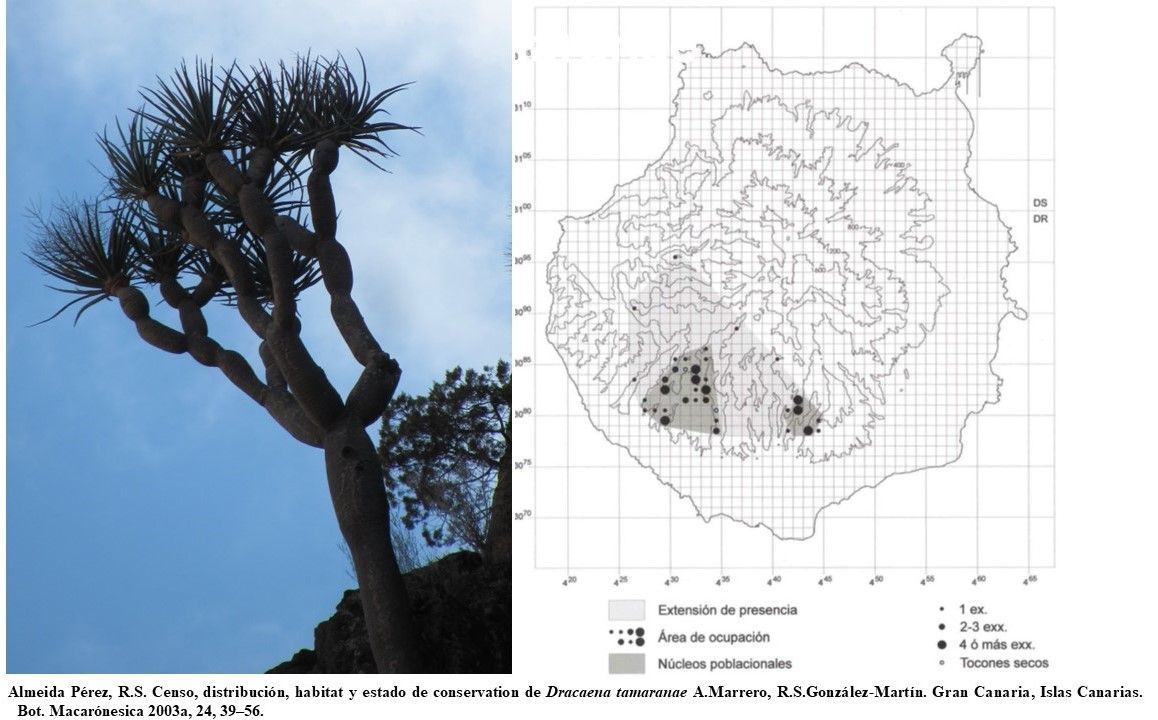
Dracaena tamaranae Marrero Rodr., R. S. Almeida & Gonz.-Mart.
D. tamaranae is a cliff-dwelling species restricted to Gran Canaria Island, where it grows on inaccessible slopes between 342 and 1270 m a.s.l. in the southwestern region. The whole population of this endemic species of Grand Canaria (Canary Islands), consisted of 86 trees. Although the sites with D. tamaranae tend to be shady and humid, they are more xeric and hotter when compared with sites inhabited by D. draco. The average annual rainfall in the species altitudinal range is between 200 and 400 mm and the leeward southwestern region of Gran Canaria is not influenced by moisture-bearing trade winds. Rains in the area tend to be torrential and very irregular, usually occuring between November and February, making the rest of the year a dry period. The average annual temperature does not reach 21O C; however, the monthly averages exceed 25O C in some summer months and they rarely fall below 15O C in the winter. This arid and semiarid climate determines a pronounced xerophytic character of D. tamaranae communities that belong to the class Rhamno crenulatae–Oleetea cerasiformis, and, to a lesser extent, to Kleinio–Euphorbietea canariensis (at lower elevations) and Chamaecytiso–Pinetea canariensis (at higher elevations).
Northeast African/Arabian group
The species of the northeast African/Arabian group (D. cinnabari, D. serrulata, D. ombet s.l., and D. ellenbeckiana) are found in the arid and semiarid vegetation of mountains and escarpments along the Red Sea and Gulf of Aden and on Socotra Island. The dragon trees grow in higher elevations and are strongly dependent on orographic fog formation due to the high aridity, which brings additional moisture into the environment. The horizontal precipitation constitutes an important input of water which may exceed the rainfall and localities with the dragon trees are commonly found in areas known as mist oases.
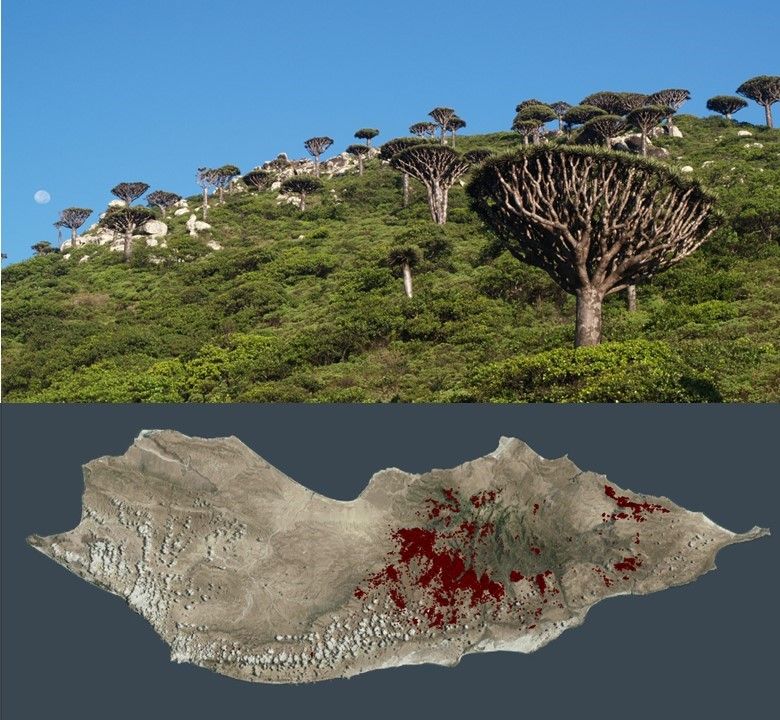
Dracaena cinnabari Balf. f.
D. cinnabari, endemic species to Socotra, in the whole of Socotra, up to the elevational limit of 1100 m a.s.l., a total of 80,134 individual D. cinnabari trees were detected, while using the combination of object-based classification with manual vectorization of remote sensing data. The total area of occupancy was estimated as 519.63 km2. The species is confined to areas subject to the formation of the monsoon mists and clouds, mostly to limestone plateaus and mountains, where the tree profits from its ability to intercept horizontal precipitation. The rocky limestone plateaus are located between 400 and 700 m a.s.l. and the D. cinnabari grows here as an emergent in open semi-evergreen Buxanthus pedicellatus–Dracaena cinnabari woodland. The montane and high-montane vegetation with D. cinnabari is found on limestone foothills and granite peaks above 700 m a.s.l., where the temperature drops during the late afternoon and evening leads to the regular formation of clouds. The interception of horizontal precipitation from clouds and monsoon mist together with dewfall substantially enhance the available moisture leading to the formation of lush vegetation where dragon trees grow as scattered emergents overlooking a dense species-rich shrub layer. The findings indicate that D. cinnabari acts as an ecosystem engineer, i.e., a key species that alters the abiotic environment, controls the availability of resources, and facilitates the growth of other species.d, and it is transgressing into Acacia–Commiphora deciduous bushland; altitudinal range 1000–1500 m a.s.l.; and, mean annual rainfall range 300–1000 mm.
Dracaena ombet subsp. ombet Baker
D. ombet subsp. ombet is mentioned from Egypt, Djibouti, Eritrea, Ethiopia, Somalia, Sudan, and Saudi Arabia. The species inhabits the sea-facing mountains along the Red Sea and north-ethiopian escarpment, mostly in areas that are affected by the mists that come with the winds. On the north edge of its distribution, it grows in desertic igneous mountains between 450 and 950 m a.s.l. with 50 mm of annual rainfall and with up to 400 mm of additional water per year from mist and cloud. It is mostly found growing as isolated individuals or in small groves on steep rocky slopes and cliffs. In the south, along the north-ethiopian escarpment, D. ombet subsp. ombet thrives in higher altitudes (1400–1800 m a.s.l.), in the transition zone between Afromontane vegetation and Acacia–Commiphora bushland, and forms Dracaena ombet–Acacia etbaica community.
Dracaena ombet subsp. schizantha (Baker) Bos
D. ombet subsp. schizantha is part of dry single-dominant Afromontane Juniperus and Juniperus–Olea forest in the east-ethiopian escarpment where it grows, especially along the forest edges. Further east, it grows in Buxanthus pedicellatus–Acokanthera schimperi bushland and in Rhigozum somalense bushland.
Dracaena serrulata Baker
D. serrulata is found in areas with Acacia-Commiphora bushland in sea-facing mountains, including their inner regions, along the southwestern edge of the Arabian Peninsula (Oman, Yemen, Saudi Arabia). The average annual rainfall in areas with D. serrulata is approximately 200 mm.
Dracaena ellenbeckiana Engl.
D. ellenbeckiana generally grows on rocky slopes in evergreen and semi-evergreen bushlands or open dry woodlands in elevations between 1000 and 2100 m a.s.l.. In Ethiopia, it is known from the transitional vegetation between dry Afromontane forest (Juniperus and Juniperus–Olea forest) and East African evergreen bushland, from typical East African evergreen bushland, and it is transgressing into Acacia–Commiphora deciduous bushland; altitudinal range 1000–1500 m a.s.l.; and, mean annual rainfall range 300–1000 mm.
Southeast Asian group
The southeast Asian Dracaena species (D. cambodiana, D. cochinchinensis, D. jayniana, D. kaweesakii, and D. yuccifolia) belonging into the dragon tree group grow in tropical or subtropical areas with higher rainfall than the previous groups, but they are usually associated with cliffs and the tops of limestone rocks in karstic landscapes, where water availability is reduced due to relief and edaphic constraints.
Dracaena jayniana Wilkin & Suksathan.
Eight populations of D. jayniana were found in Thailand, with a calculated area of occupancy of 32 km2. D. jayniana is a species of highly seasonal deciduous vegetation of limestone karst and it is usually found on hilltops rather than on steep cliffs; it grows in elevations between 300 and 500 m a.s.l.
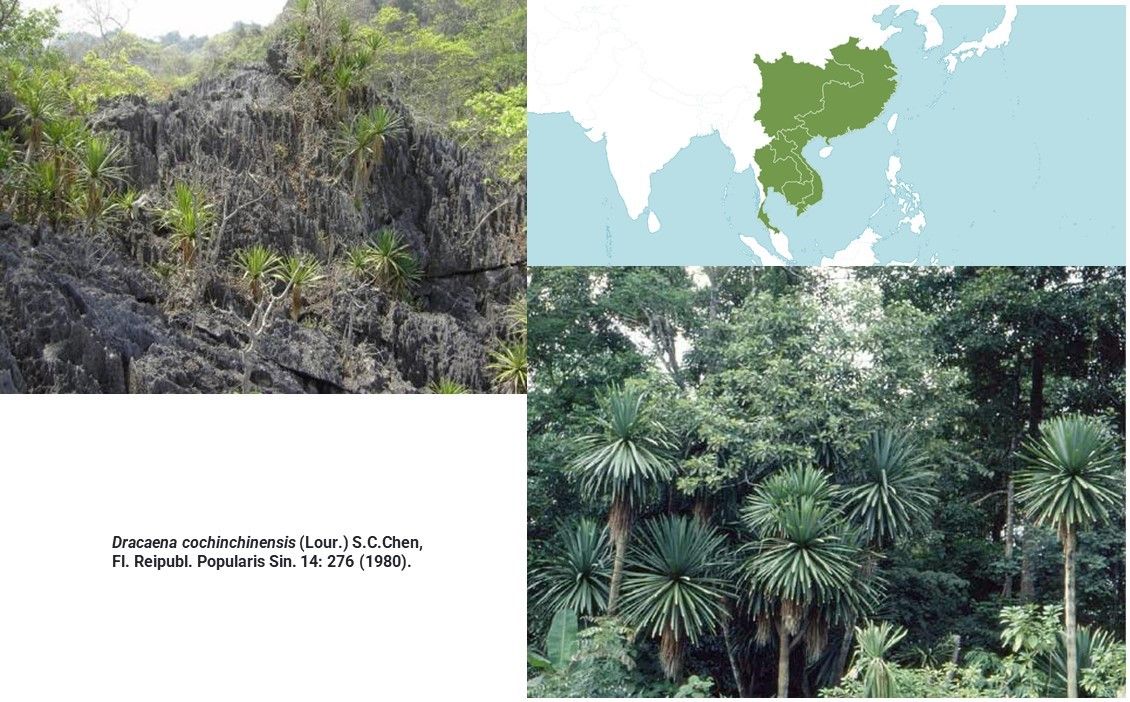
Dracaena cochinchinensis (Lour.) S.C.Chen
D. cochinchinensis is a species of tropical seasonal evergreen and semi-evergreen moist forests, where it grows on limestone slopes in higher elevation. It grows in environment where seasonal dryness is partially compensated by dense fog and low temperatures during the dry season.
Dracaena yuccifolia Ridl.
The native range of this species is from Peninsula Thailand to Peninsula Malaysia. It is a shrub and grows primarily in the wet tropical biome.
Dracaena cambodiana Pierre ex Gagnep.
Only 10 populations of D. cambodiana remain on Hainan Island; they grow in distinct habitats and have almost all been seriously isolated. The population sizes range from 20 to 5000 individuals in total. The abundance of whole population of D. cambodiana was estimated on Hainan Island to be 7,590 individuals. D. cambodiana is usually found in escarpments of island-like limestone mountains. However, it has also been documented growing on granite along the coast with low rainfall, dry heat, and soil with weak water storage capacity as well as along streams in the mountains far from the sea, which suggests that D. cambodiana can grow in ecologically diverse habitats.
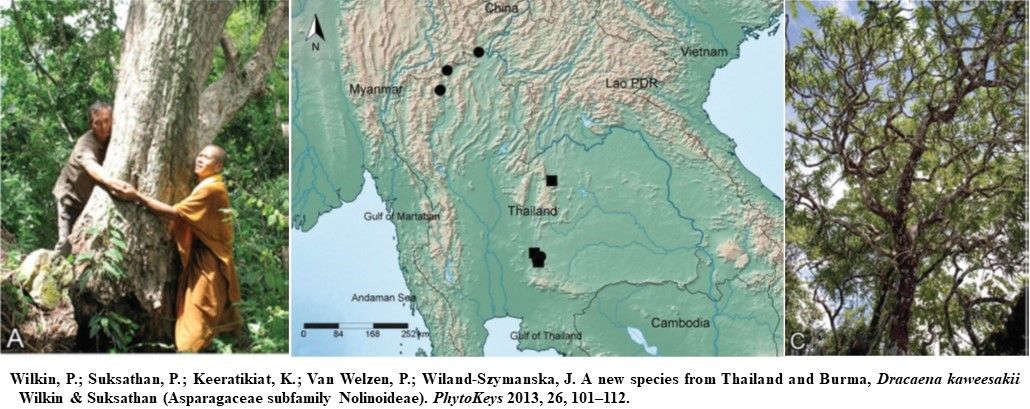
Dracaena kaweesakii Wilkin & Suksathan
D. kaweesakii of Thailand and Myanmar was estimated to be 2,500 mature individuals, with an area of occupancy of 44 km2. Individual populations contain tens to hundreds of specimens. D. kaweesakii is a species of limestone rock and it grows between 550 and 2000 m a.s.l.

Contact
prof. Petr Maděra
Department of Forest Botany, Dendrology and Geobiocoenology
faculty of Forestry and Wood Technology
Mendel University in Brno
petrmad@mendelu.cz
© Dragon Tree Consortium 2023 | web rostanetek | admin